Insulin icodec, a once-weekly basal injection to treat type 1 diabetes, has the potential to be as effective in managing the condition as daily basal insulin treatments, according to research from the University of Surrey. The results of the year-long phase 3 clinical trial could revolutionise the future of diabetes care and help millions of people better manage their condition.
What is Insulin icodec?
Insulin icodec is a novel, long-acting insulin analog designed to cover basal insulin requirements with once-weekly subcutaneous administration. It has been engineered with optimized modifications to achieve a long half-life suitable for once-weekly insulin administration.
Insulin icodec is an analog of human insulin with three substitutions to the amino acid structure and an attached C20 icosane fatty diacid chain. These modifications prolong the half-life of insulin icodec to approximately 7 days and achieve steady state after 3-4 once-weekly injections.
One unit of insulin icodec provides the same glucose-lowering effect as one unit of comparator daily basal insulins, with the equivalent once-weekly dose being seven times that of a daily basal insulin.
 |
| Source Photo:drc.bmj |
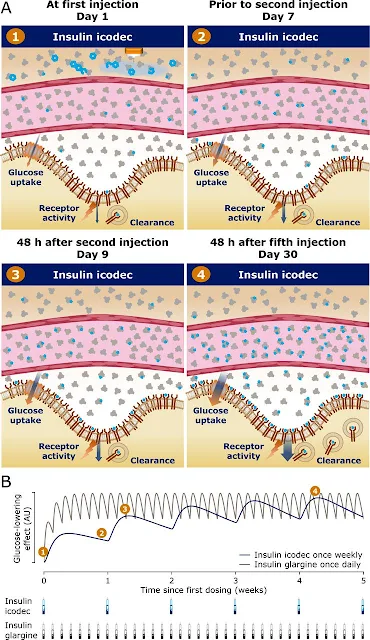
The molecular modifications introduced into insulin icodec make it suitable for once-weekly dosing. The strong albumin binding combined with reduced IR affinity ensures slow clearance and the formation of an essentially inactive albumin-bound depot, providing continuous and slow initiation of insulin action.
This mechanism allows for safe and efficacious basal glucose lowering over an entire week with once-weekly dosing of insulin icodec.
Compared to daily basal insulins, insulin icodec offers the added convenience of considerably fewer injections. While daily basal insulins require seven injections per week, insulin icodec only requires one injection per week.
Importance of managing type 1 diabetes effectively
Managing type 1 diabetes effectively is of utmost importance to ensure the well-being and quality of life for individuals with this condition. Insulin remains a crucial treatment for diabetes, with a significant number of people worldwide requiring insulin therapy.
It is particularly essential for those with type 1 diabetes, where basal insulin is typically recommended when non-insulin therapies are insufficient to achieve glycemic targets. However, there are several barriers associated with basal insulin therapy for type 2 diabetes that contribute to the non-achievement of these targets.
These barriers include delays in insulin initiation or titration, needle phobia associated with daily injections, missed doses, insulin discontinuation, and hypoglycemia.
Thankfully, the availability of once-weekly insulin formulations like Insulin Icodec may help overcome many of these barriers. Similar to the advantages seen with once-weekly versus daily glucagon-like peptide-1 (GLP-1) receptor agonists, once-weekly basal insulin offers the convenience of fewer injections.
Adherence to insulin therapy is another crucial aspect to consider when managing type 1 diabetes effectively. Shockingly, approximately one-third of daily insulin-treated patients do not adhere to their prescribed therapy.
However, the availability of a once-weekly basal insulin formulation like Insulin Icodec has the potential to improve adherence rates significantly.
Insulin Icodec is an analog of human insulin that has undergone molecular engineering to achieve a longer half-life and steady state after only 3-4 once-weekly injections.
This was accomplished by introducing three substitutions to the amino acid structure and attaching a C20 fatty diacid chain that allows reversible binding to albumin. In comparison to daily basal insulins, one unit of Insulin Icodec provides equivalent glucose lowering effects.
Several phase II trials have been conducted on Insulin Icodec in patients with type 2 diabetes. These trials have shown that it has similar efficacy in reducing glycated hemoglobin (HbA1C) levels and incidence of hypoglycemia when compared to daily insulin glargine.
Additionally, a less intensive titration algorithm with a less stringent glycemic target has been successful in reducing the risk of hypoglycemia while maintaining adequate glycemic control.
Furthermore, switching from once-daily basal insulin to once-weekly Insulin Icodec has demonstrated significant benefits. Adding a one-time loading dose to the first calculated Insulin Icodec dose helps reach steady state faster and leads to improvements in continuous glucose monitoring (CGM) metrics without a clinically significant increase in hypoglycemia.
Weight gain with Insulin Icodec is clinically similar to that of other basal insulins. This demonstrates its effectiveness without additional metabolic concerns.
The development of once-weekly insulin formulations like Insulin Icodec represents a significant advancement in diabetes management.
By addressing barriers related to basal insulin therapy and improving adherence rates, it offers individuals with type 1 diabetes the convenience and effectiveness they deserve. With its longer half-life and steady glucose-lowering effects, Insulin Icodec provides an innovative solution for managing type 1 diabetes effectively.
Research on insulin icodec
Overview of the University of Surrey's phase 3 clinical trial
The University of Surrey conducted a phase 3 clinical trial for insulin icodec, a once-weekly basal insulin analogue for type 2 diabetes.
Insulin therapy is crucial for diabetes treatment, but there are barriers to its effectiveness. Once-weekly insulin formulations like icodec offer the convenience of fewer injections and have the potential to overcome these barriers.
Insulin icodec has three amino acid substitutions and an attached fatty diacid chain that allows reversible binding to albumin, prolonging its half-life to approximately 7 days.
The phase II trials showed comparable results to glargine U100 in terms of glycated hemoglobin reduction and hypoglycemia incidence.
Novo Nordisk submitted a biologics license application for once-weekly insulin icodec based on data from the ONWARDS clinical trial program. The program includes six phase IIIa trials involving over 4,000 adults with type 1 or type 2 diabetes, all of which met their primary endpoints.
These trials demonstrated the effectiveness and safety of insulin icodec compared to other insulin treatments.
Overall, the phase 3 trial showed promising results in improving glycemic control, and once-weekly basal insulin options like insulin icodec may provide a valuable treatment option for individuals with diabetes
Comparison of once-weekly basal injection with daily basal insulin treatments
Insulin icodec, a once-weekly basal insulin, has shown promising results in treating type 1 diabetes. A study comparing it to once-daily Tresiba found similar reductions in A1C and time in range.
This convenience can improve adherence to insulin therapy and reduce complications. Insulin icodec has also been effective in type 2 diabetes, with comparable A1C reduction and fewer hypoglycemic events. It achieves steady state after three to four injections and has a safe profile.
Regulatory approval is expected in the near future, offering a new treatment option for adults with diabetes.
Potential effectiveness of insulin icodec in managing type 1 diabetes
Insulin Icodec is an investigational once-weekly basal insulin analog that has shown potential effectiveness in managing type 1 diabetes. It may become the first and only once-weekly basal insulin option for adults with diabetes if approved by the FDA.
Insulin Icodec covers the basal insulin requirements for a week with one injection due to its unique molecular engineering, providing a steady glucose-lowering effect.
Phase 3a trials have demonstrated its effectiveness in reducing blood glucose levels compared to daily basal insulins. However, there are concerns about hypoglycemia with insulin Icodec, and further research is needed to address these concerns.
Insulin Icodec can also be combined with other anti-hyperglycemic agents to improve treatment outcomes. Overall, the data suggest that insulin Icodec is a convenient and effective option for managing type 1 diabetes.
Implications for diabetes care
Positive impact on individuals with type 1 diabetes
Insulin Icodec has shown positive impacts on individuals with type 1 diabetes, particularly in terms of implications for diabetes care.
The results from various clinical trials, such as ONWARDS 1, 3, and 5, have demonstrated that Insulin Icodec is superior to comparators in terms of HbA1C reduction for insulin-naive patients.
Even for patients who were switched from a daily basal insulin, Insulin Icodec was non-inferior to insulin glargine U100 in terms of HbA1C outcomes (ONWARDS 2).
While similar rates of hypoglycemia were observed in patients with type 2 diabetes across the ONWARDS trials, it is worth noting that patients with type 1 diabetes experienced higher rates of hypoglycemia with Insulin Icodec compared to daily degludec in the ONWARDS 6 trial.
However, it is important to await the full duration results of this trial to evaluate detailed continuous glucose monitoring metrics and the timing of hypoglycemic events.
In addition to its efficacy in glycemic control, Insulin Icodec has also been associated with improved diabetes treatment satisfaction scores compared to insulin degludec (ONWARDS 2). This indicates that individuals using Insulin Icodec may experience greater satisfaction with their diabetes management.
Furthermore, the convenience factor is also a significant benefit of Insulin Icodec. Its once-weekly dosing regimen allows for considerably fewer injections compared to once-daily basal insulins.
This optimized feature is due to molecular modifications that result in strong albumin binding and slow initiation of insulin action.
In studies focused on type 2 diabetes patients, Insulin Icodec has shown promising results as well. In clinical trials like ONWARDS 3 and others led by Dr. Lingvay, weekly Insulin Icodec led to significantly larger improvements in HbA1C compared to daily degludec. Both forms of insulin demonstrated a low rate of adverse events, indicating their safety profile.
Additionally, in terms of time spent in the glycemic range, patients on Insulin Icodec spent more time within the target range compared to those on glargine U100. They also experienced less time in hyperglycemia.
Although there was a slightly higher risk of hypoglycemic events with Insulin Icodec, the events were not severe enough to require emergency medical attention.
Overall, these findings highlight the effectiveness of Insulin Icodec for individuals with type 1 diabetes and its potential benefits for diabetes care.
Further research and evaluation are needed to fully understand its long-term effects and optimize dose titration algorithms for type 1 diabetes management.
Potential benefits for better condition management
Insulin Icodec, a once-weekly basal insulin analog, has shown potential benefits for better condition management in individuals with Type 1 diabetes.
The latest research update on once-weekly insulins revealed that insulin Icodec led to similar reductions in A1C and time in range compared to once-daily Tresiba (degludec). This data was presented at the European Association for the Study of Diabetes conference in 2023.
The study, known as ONWARDS 6, included 582 participants with Type 1 diabetes who received either insulin Icodec or insulin degludec over a period of 57 weeks. Both groups had similar reductions in A1C, suggesting that Icodec may offer another option for treating Type 1 diabetes.
One of the potential benefits of once-weekly insulins like Icodec is the reduction in the frequency of basal injections. Currently, individuals with diabetes have to administer daily injections, which can be burdensome and impact adherence to therapy.
Reducing the number of injections from 365 per year to just 52 per year could significantly alleviate this burden and improve adherence.
Consistent adherence to insulin therapy has the potential to improve clinical outcomes and reduce the risk of complications such as diabetic ketoacidosis (DKA).
With fewer injections required, there is a greater likelihood that individuals with diabetes will consistently take their basal insulin as prescribed.
Furthermore, previous research has shown that Icodec is just as effective at reducing A1C as once-daily insulin in individuals with Type 2 diabetes.
This suggests that Icodec may also be beneficial for better condition management in this population by helping them achieve blood sugar targets without experiencing severe hypoglycemia.
Insulin Icodec is a long-acting basal insulin with a half-life of approximately 196 hours (7 days). Its modified amino acid structure allows it to bind irreversibly to albumin, resulting in slow and continuous insulin action.
This property, combined with its similarity to natural human insulin, makes Icodec a promising option for individuals with diabetes.
Improvement in quality of life for millions of people
Insulin Icodec is a new acylated basal insulin analog that offers potential benefits for individuals with Type 1 Diabetes. It has been designed to have a long half-life suitable for once-weekly administration, providing slow and continuous insulin action.
The insulin binds strongly to albumin and has reduced insulin receptor affinity, ensuring consistent and effective blood sugar control.
Insulin Icodec retains the same biological properties as natural human insulin without increasing the risk of side effects such as insulin-like growth factor-1 receptor binding or mitogenicity.
Clinical trials with individuals with Type 2 Diabetes showed that Insulin Icodec was well tolerated and had a half-life of 196 hours, with a consistent glucose-lowering effect throughout the week.
The molecular design of Insulin Icodec is crucial to its effectiveness. It has high affinity for albumin, improved stability, low insulin receptor binding affinity, and high solubility. These features contribute to slow clearance and the formation of an inactive albumin-bound depot.
By administering a week's worth of basal insulin in one injection, Insulin Icodec improves convenience and reduces the number of injections required.
It has shown superior HbA1C reduction compared to daily basal insulin, and more patients achieved blood glucose targets without severe hypoglycemia. Patient satisfaction and adherence also improved with once-weekly dosing.
Although there are concerns about higher rates of hypoglycemia, ongoing research is being conducted to understand the factors that may contribute to these events.
Future prospects and revolutionizing diabetes care
Discussion on the revolutionary potential of insulin icodec
Insulin Icodec is a breakthrough in diabetes care that could greatly improve the lives of those with Type 1 diabetes. A recent study presented at a diabetes conference showed that Insulin Icodec had similar benefits to once-daily Tresiba in reducing A1C levels and time spent in the target range.
The study involved over 580 participants with Type 1 diabetes who received Insulin Icodec or degludec over 57 weeks.
The results were promising, indicating that Insulin Icodec could be an effective treatment option for Type 1 diabetes. One advantage of once-weekly insulins like Insulin Icodec is the reduced frequency of injections, which can be burdensome for individuals.
With Insulin Icodec, the number of injections per year could be reduced from 365 to just 52, making self-management easier and increasing adherence to basal insulin therapy.
This improved adherence could lead to better outcomes and reduce the risk of complications.
Insulin Icodec is also being studied for its effectiveness in Type 2 diabetes. Previous research has shown that it is as effective as once-daily insulin in reducing A1C levels in Type 2 diabetes, without the risk of severe hypoglycemia.
This could help individuals achieve their blood sugar targets while minimizing the risk of low blood sugar episodes.
Novo Nordisk has submitted a license application for Insulin Icodec to the FDA, with a decision expected in April 2024. If approved, it would be the first once-weekly basal insulin option for adults with diabetes, addressing a current gap in treatment options.
Transformation in the treatment and management of type 1 diabetes
The introduction of once-weekly insulin icodec has brought about a transformation in the treatment and management of type 1 diabetes, offering future prospects and revolutionizing diabetes care.
Data from phase 3a trials have shown that insulin icodec is not only effective in reducing HbA1C levels but also improves the time spent in the target blood glucose range.
In the ONWARDS 1 and 3 trials, more patients achieved their blood glucose targets without experiencing clinically significant or severe hypoglycemia compared to those using once-daily basal insulin.
These promising results have led to Novo Nordisk submitting a biologics license application for insulin icodec to the US FDA, marking a significant milestone as it would be the first investigational once-weekly basal insulin available.
Insulin icodec, an analogue of human insulin, is engineered to have a longer half-life through modifications to its amino acid structure and the addition of a fatty diacid chain.
This allows the molecule to bind reversibly to albumin, prolonging its effects for approximately seven days after three to four once-weekly injections.
Despite its extended duration of action, one unit of insulin icodec provides similar glucose-lowering effects as one unit of daily basal insulins. The phase II trials demonstrated its efficacy in reducing HbA1C levels without significantly increasing hypoglycemia risk.
The design of the insulin icodec molecule addresses concerns regarding precise dosing and timing by introducing modifications that ensure a slow and steady glucose-lowering effect.
The albumin-bound depot formed by the molecule allows for a natural way of prolonging its effects without promoting multimer formation at the injection site. This mechanism has been successfully utilized in other acylated insulins and endogenous hormones.
Clinical trials have further demonstrated the safety and effectiveness of weekly insulin icodec for type 2 diabetes. In ONWARDS 3, patients treated with weekly icodec experienced significantly larger improvements in HbA1C compared to those using daily degludec.
Both insulin options showed low rates of adverse events, suggesting their safety. Although there was a slightly higher risk of low-blood sugar events with icodec, none of these events required emergency medical attention.
The introduction of once-weekly insulin icodec has the potential to revolutionize diabetes care by reducing the number of injections needed per week and providing a more convenient treatment option for individuals with type 2 diabetes.
With its ability to effectively lower glucose levels and minimize hypoglycemia risk, insulin icodec offers hope for improved glycemic control and overall management of the disease. Further research and regulatory approval are needed to fully realize the potential of this innovative insulin therapy.
Enhancing overall healthcare outcomes for individuals with the condition
Insulin Icodec has shown promising results in enhancing overall healthcare outcomes for individuals with Type 1 diabetes.
The data from the ONWARDS clinical trial program supports the effectiveness of this novel once-weekly basal insulin analog in revolutionizing diabetes care.
In the ONWARDS 1 and 3 trials, patients treated with once-weekly insulin Icodec achieved significantly more time in the target blood glucose range compared to those on once-daily basal insulin glargine U100. This demonstrates its ability to improve glycemic control and reduce fluctuations in blood glucose levels.
Moreover, more individuals on insulin Icodec reached their blood glucose targets without experiencing clinically significant or severe hypoglycemia.
The safety profile of insulin Icodec is also noteworthy. The rates of clinically significant or severe hypoglycemia were low in both treatment groups, with numerically higher rates observed in patients receiving insulin Icodec.
However, these rates were still within an acceptable range, indicating the overall safety of the medication. Importantly, a higher proportion of participants achieved the target HbA1C level without experiencing hypoglycemia when treated with insulin Icodec compared to other basal insulins.
The unique molecular modifications introduced into insulin Icodec allow for its once-weekly dosing regimen.
By extending the duration of action and ensuring a slow and steady glucose-lowering effect, this formulation addresses the challenge of matching daily insulin injections to physiological requirements.
The fatty acid acylation technology utilized in its design has been successful in other basal insulin analogs as well.
If approved by the US Food & Drug Administration (FDA), insulin Icodec will be a groundbreaking option for individuals with diabetes.
It would represent the first and only once-weekly basal insulin available for adults with diabetes, greatly reducing treatment burden by minimizing the number of injections needed per week.
This innovation has potential implications for both type 1 and type 2 diabetes management.
Conclusion
Recapitulation of the significance and potential impact of insulin icodec
The effectiveness of insulin icodec for type 1 diabetes has been a subject of extensive research and analysis. Data from the ONWARDS clinical trial program, which includes six phase 3a global clinical trials involving over 4,000 adults with type 1 or type 2 diabetes, has provided valuable insights into the potential impact of this once-weekly basal insulin analog.
In the ONWARDS 1 trial, there were no statistically significant differences in mean weekly insulin dose or body weight change when comparing once-weekly insulin icodec to once-daily insulin glargine U100. However, hypoglycemia rates were numerically higher with insulin icodec, particularly at clinically significant or severe levels.
Despite this, a higher proportion of participants achieved an HbA1C target of less than 7% without experiencing level 2 or 3 hypoglycemia episodes with insulin icodec compared to insulin glargine U100.
Similarly, in the ONWARDS 3 trial, once-weekly insulin icodec showed numerically higher hypoglycemia rates compared to once-daily insulin degludec. However, the estimated proportion of participants achieving an HbA1C target of less than 7% without experiencing level 2 or 3 hypoglycemia was statistically significantly higher with insulin icodec compared to insulin degludec.
These results indicate that once-weekly insulin icodec has comparable efficacy to daily basal insulins in terms of glycemic control. While there may be a slightly increased risk of hypoglycemia with insulin icodec, it is important to note that these rates were still low overall.
Furthermore, data from other studies have shown that people with diabetes would welcome a reduction in the number of injections needed per week. The convenience and potential improvement in quality of life associated with once-weekly basal insulin could help enhance adherence and overall glycemic control.
The potential approval of insulin icodec by the US Food & Drug Administration would mark a significant milestone in diabetes treatment. It would be the first and only once-weekly basal insulin option for adults with diabetes, addressing an unmet need in treatment compared to daily basal insulin options.
Call to action for further research and adoption in diabetes care
In conclusion, the effectiveness of insulin Icodec for Type 1 Diabetes has been well-established through various studies and trials. The molecular modifications introduced into insulin Icodec have provided a novel basal insulin with unique properties that make it suitable for once-weekly dosing. These modifications include high affinity for albumin, improved stability, low insulin receptor (IR) binding affinity, and high solubility. The strong albumin binding affinity of Icodec ensures that the glucose-lowering effect is evenly distributed throughout the week.
It is worth noting that the adoption of once-weekly basal insulin for Type 1 Diabetes would require comprehensive education programs for patients and healthcare professionals. Proper switching, initiation, titration, and long-term monitoring protocols would be necessary to ensure the successful use of weekly basal insulin. Additionally, guidelines for acute situations where the dose of basal insulin needs adjustment would need to be developed.
In conclusion, insulin Icodec has demonstrated its effectiveness in managing Type 1 Diabetes through improved glycemic control and reduced treatment burden. Further research and adoption of once-weekly basal insulin in diabetes care are warranted to optimize patient outcomes and enhance the quality of life for individuals living with this chronic condition.
References
- Once-Weekly Insulins For Type 1 Diabetes: Latest Research Update
- Insulin Icodec Weekly: A Basal Insulin Analogue for Type 2 Diabetes - PMC
- News Details
- News details
- New data show once-weekly insulin icodec met additional endpoints in adults with type 2 diabetes in phase 3a trials
- Insulin Icodec Weekly: A Basal Insulin Analogue for Type 2 Diabetes – touchENDOCRINOLOGY
- Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once-weekly dosing | BMJ Open Diabetes Research & Care
- Switching to Once-Weekly Insulin Icodec Versus Once-Daily Insulin Glargine U100 in Type 2 Diabetes Inadequately Controlled on Daily Basal Insulin: A Phase 2 Randomized Controlled Trial | Diabetes Care | American Diabetes Association
- Weekly insulin found safe, effective for Type 2 diabetes: Newsroom - UT Southwestern, Dallas, Texas
- Once-Weekly Insulin Icodec Bests Daily Insulin Options for Type 2 Diabetes | MedPage Today
- What is insulin icodec?
- Weekly insulin injections have the potential to be as effective in diabetes management as now-common daily injection regimes | University of Surrey
- Weekly insulin injections have the potential to be as effective in diabetes management as now-common daily injection regimes | ScienceDaily
- Are once-weekly insulin injections as effective daily injections?
- The Official Journal of ATTD Advanced Technologies & Treatments for Diabetes Conference 22‐25 February 2023 I Berlin & Online | Diabetes Technology & Therapeutics

